What are Redox Reactions?
A redox reaction means a compound reaction where electrons are transferred between two reactions doing it. This procedure of transferring electrons can be identified by observing the changes in the oxidation states in the reacting species.
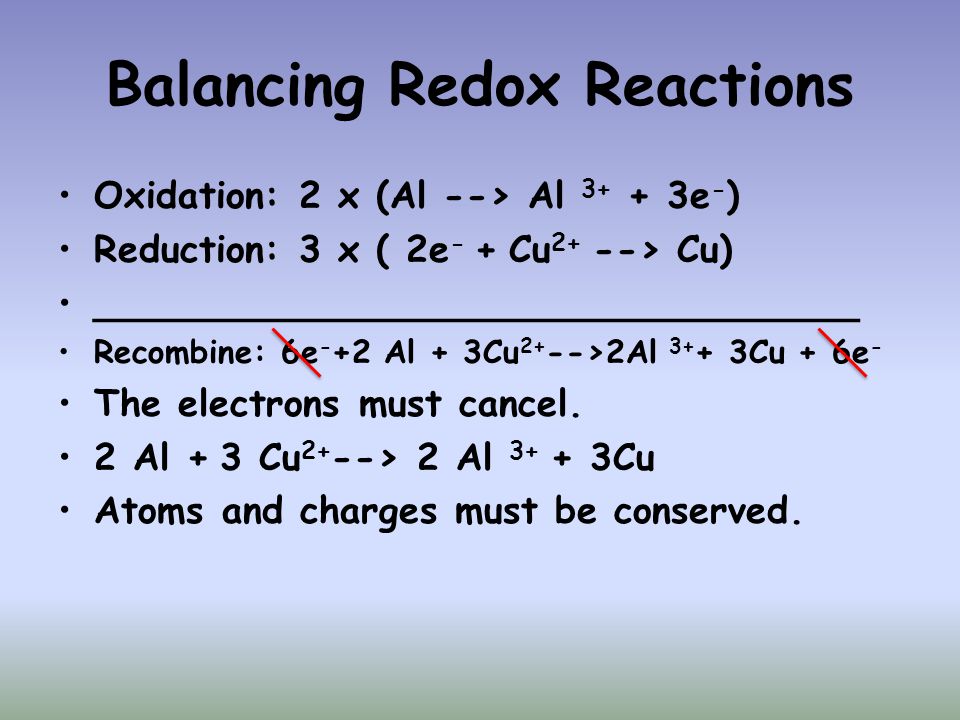
Loosing electrons and also the corresponding boost in the oxidation condition of the reactant is called oxidation. The gain of electrons as well as the corresponding loss of your the reactant is called reduction.
The electron-accepting species that undergo a reduction in redox reactions are called oxidizing agents. The electron-donating species that usually give electrons are called reducing agents.
Redox Reaction Calculator: Sometimes you may feel it is complex to unravel the redox reaction for any chemical reaction? In that case, then utilise online handy calculator as it does lengthy calculations and provides the precise answer inside a fraction of seconds.
Steps to Calculate Redox Reaction
Below-mentioned could be the step by step process on how to solve the redox result of a compound reaction.
Get the standard potential, the quantity of electrons transferred through the reaction, temperature, and ratio with the concentration of product versus reactant details.
Divide temperature from the quantity of electrons transferred.
Discover the log with the ratio from the energy product versus reactant value.
Multiply the division result by the log result value.
Subtract the obtained product from standard possible ways to understand the redox reaction value.
For more info about Redox calculator you can check our web page
